|
(Files in red–history) |
Ions in Chemistry
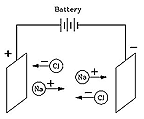
But how, and why? The answer was given in 1884 by Svante Arrhenius (1859-1927), a many-talented Swede who received the 1903 Nobel prize for chemistry and who (among his many achievements) first suggested the "greenhouse effect." Arrhenius proposed that when a compound like table salt NaCl (sodium chloride) was dissolved in water, it broke up into electrically charged "ions" (Greek for "the ones that move") Na+ and Cl-. Electric forces made Na+ ions move in one direction, Cl- ions in the opposite one, and that was how the electric current was carried.
Although at first this seemed like a strange idea, today it is quite well understood. Many chemical molecules are formed when atoms share electrons, but molecules such as those of NaCl are different. There, the sodium atom (Na) gives up an electron to the chlorine (Cl), creating ions Na+ and Cl-, which in solid salt are held together by their electric attraction ("ionic bond"). Water, however, greatly weakens that attraction (on a microscopic scale), allowing the ions to drift free whenever salt is dissolved in water, and allowing the water to conduct electricity.
|